Marshmallow Molecules
By Lori Altenbaumer, 7th/8th Math & Science
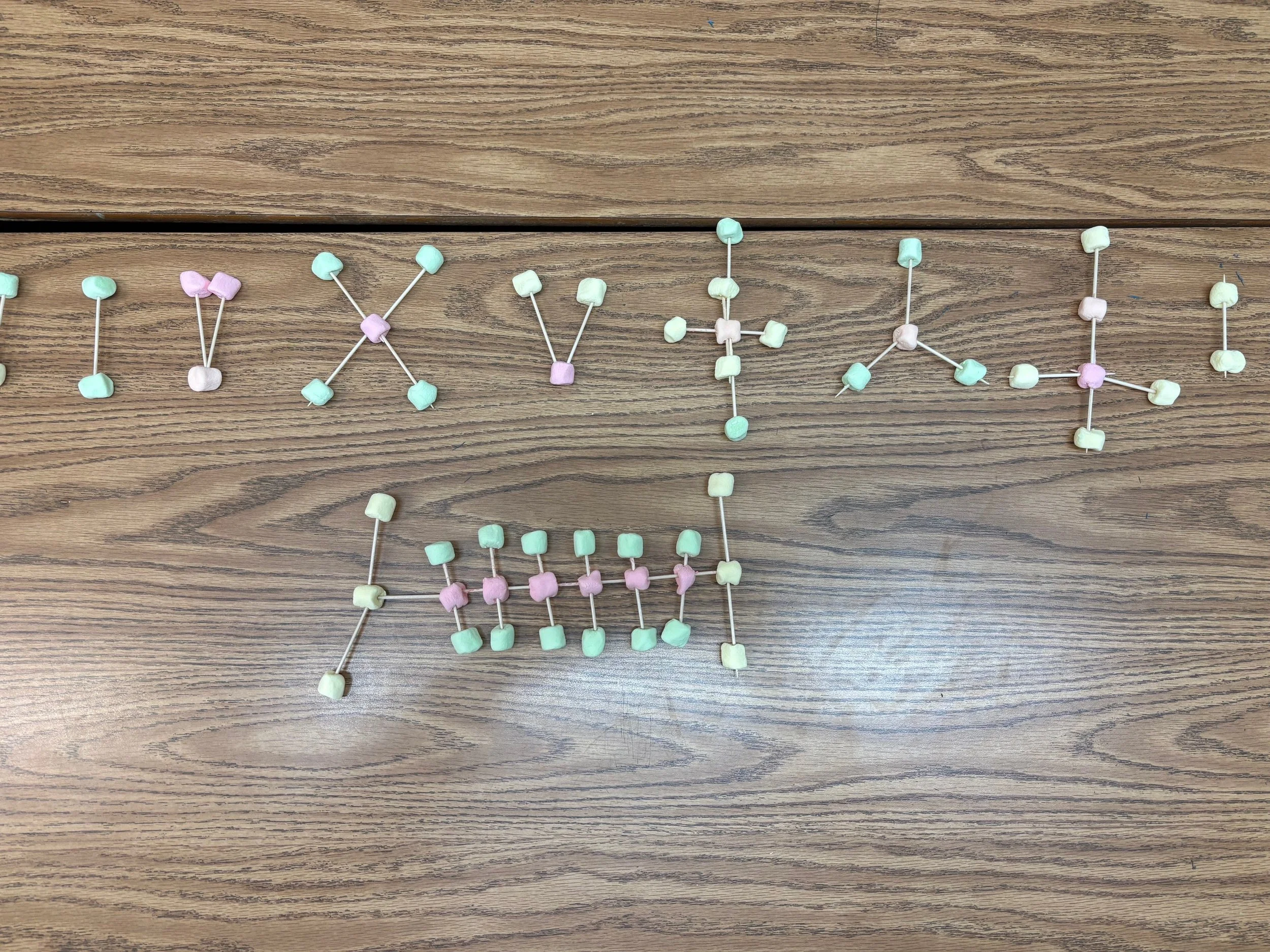
This week, our 7th grade scientists dove into the world of chemistry with our Marshmallow Molecules Lab, a hands-on activity designed to help students truly see and build what chemical formulas represent. Using mini marshmallows and toothpicks, students transformed abstract symbols and subscripts into colorful, three-dimensional molecular models—making chemistry both engaging and memorable.
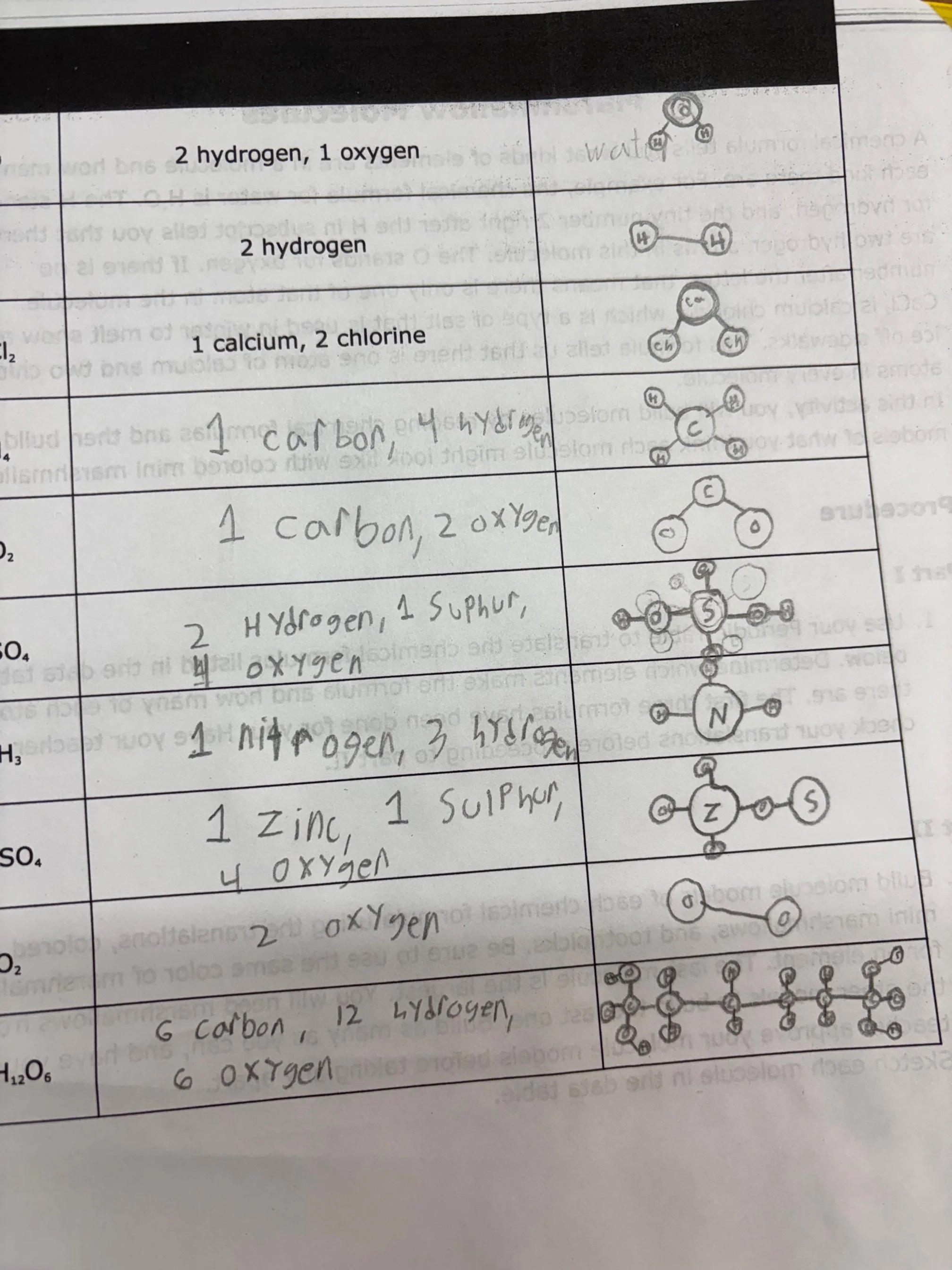
Before building, students worked through Part I of the lab by carefully translating chemical formulas using their periodic tables. They identified which elements were present and how many atoms of each were required, paying close attention to subscripts and what they signify . This step was critical in reinforcing that a chemical formula is more than just letters—it’s a precise recipe for a molecule.
In Part II, the real fun began. Each element was assigned a specific marshmallow color, and students used toothpicks to represent chemical bonds. As they constructed molecules such as water (H₂O), carbon dioxide (CO₂), methane (CH₄), ammonia (NH₃), and calcium chloride (CaCl₂), students quickly realized that molecules can have very different shapes and sizes—even when they seem similar on paper.
The final challenge was constructing C₆H₁₂O₆, the largest molecule in the lab. Students had to combine marshmallows from previous models and collaborate strategically to make it work. This molecule led to great discussion and curiosity as students researched what it represents—glucose, a molecule essential for living organisms.
Hands-on labs like Marshmallow Molecules remind us that learning science isn’t just about memorizing formulas—it’s about building understanding, one molecule at a time.